Home > Rare endocrine disorders > Norditropin®

Norditropin® Formulary Kit
Norditropin® (somatropin) injection is indicated for pediatric patients with growth failure due to inadequate secretion of endogenous growth hormone (GH) and Prader-Willi Syndrome; short stature associated with Noonan Syndrome, Turner Syndrome, and children born small for gestational age; idiopathic short stature; and for the replacement of endogenous GH in adults with growth hormone deficiency (GHD).
We didn't find any resources that match your selection.
Please adjust your filter(s) and try again.
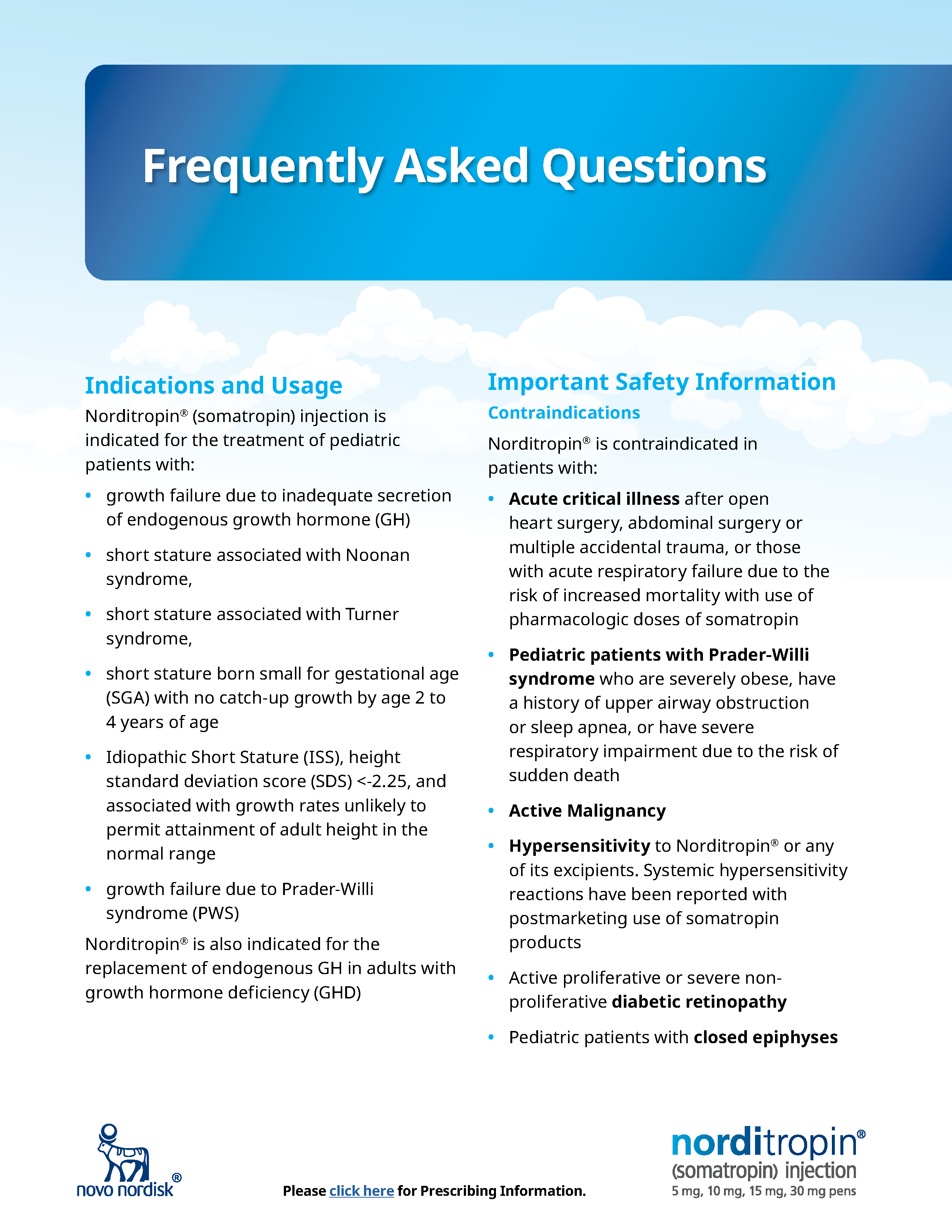
Norditropin® Frequently Asked Questions
Summary of important information about Norditropin® and FAQs about the use of Norditropin® for the treatment of certain GH disorders.

Norditropin® P&T Summary
Overview of certain GH disorders, treatment options, introduction to Norditropin®, Prescribing Information review, distribution, and administration information.

Norditropin® Product Specification Sheet
Trade unit product specification information, including wholesale acquisition cost and National Drug Code numbers, packaging information, and how Norditropin® is supplied.

Norditropin® Product Information Form for Hospitals
Similar to Prescribing Information. American Hospital Formulary Service (AHFS) format. With AHFS classification number and full Important Safety Information (ISI).

Norditropin® Full Prescribing Information
Full Prescribing Information for Norditropin®, patient product information, and instructions on how to use Norditropin® FlexPro®.

Norditropin® Professional Website
This website provides information about Norditropin®, including efficacy, pharmacology, and dosing.
Important Safety Information for Norditropin®
Contraindications
Norditropin® is contraindicated in patients with:
- Acute critical illness after open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure due to the risk of increased mortality with use of pharmacologic doses of somatropin
- Pediatric patients with Prader-Willi syndrome who are severely obese, have a history of upper airway obstruction or sleep apnea, or have severe respiratory impairment due to the risk of sudden death
- Active Malignancy
- Hypersensitivity to Norditropin® or any of its excipients. Systemic hypersensitivity reactions have been reported with postmarketing use of somatropin products
- Active proliferative or severe non-proliferative diabetic retinopathy
- Pediatric patients with closed epiphyses
Indications and Usage
Norditropin® (somatropin) injection is indicated for the treatment of pediatric patients with:
- growth failure due to inadequate secretion of endogenous growth hormone (GH)
- short stature associated with Noonan syndrome,
- short stature associated with Turner syndrome,
- short stature born small for gestational age (SGA) with no catch-up growth by age 2 to 4 years of age
- Idiopathic Short Stature (ISS), height standard deviation score (SDS) <-2.25, and associated with growth rates unlikely to permit attainment of adult height in the normal range
- growth failure due to Prader-Willi syndrome (PWS)
Norditropin® is also indicated for the replacement of endogenous GH in adults with growth hormone deficiency (GHD)
Important Safety Information
Contraindications
Norditropin® is contraindicated in patients with:
- Acute critical illness after open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure due to the risk of increased mortality with use of pharmacologic doses of somatropin
- Pediatric patients with Prader-Willi syndrome who are severely obese, have a history of upper airway obstruction or sleep apnea, or have severe respiratory impairment due to the risk of sudden death
- Active Malignancy
- Hypersensitivity to Norditropin® or any of its excipients. Systemic hypersensitivity reactions have been reported with postmarketing use of somatropin products
- Active proliferative or severe non-proliferative diabetic retinopathy
- Pediatric patients with closed epiphyses
Warnings and Precautions
- Increased mortality in patients with acute critical illness due to complications following open heart or abdominal surgery or multiple accidental trauma, or those with respiratory failure has been reported.
- Sudden death in pediatric patients with Prader-Willi Syndrome has been reported after initiating treatment with somatropin with one or more of the following risk factors: severe obesity, history of upper airway obstruction or sleep apnea, or unidentified respiratory infection. Evaluate patients for signs of upper airway obstruction and sleep apnea before initiation of treatment.
- Increased risk of neoplasms: Monitor patients with preexisting tumors for progression or recurrence. In childhood cancer survivors who were treated with radiation to the brain/head for their first neoplasm and who developed subsequent GHD and were treated with somatropin, an increased risk of a second neoplasm, in particular meningiomas, has been reported. Pediatric patients with certain rare genetic causes of short stature have an increased risk of developing malignancies and should be carefully monitored for development of neoplasms. Monitor patients carefully for increased growth, or potential malignant changes, of preexisting nevi.
- Glucose intolerance and diabetes mellitus: Treatment with somatropin may decrease insulin sensitivity, particularly at higher doses. New-onset type 2 diabetes mellitus has been reported. Monitor glucose levels in all patients. Doses of concurrent antidiabetic drugs may require adjustment.
- Intracranial hypertension has been reported in a small number of patients, usually within the first 8 weeks of somatropin treatment. Funduscopic examination should be performed before initiating treatment and periodically thereafter.
- Severe hypersensitivity: Serious systemic hypersensitivity reactions including anaphylactic reactions and angioedema have been reported with postmarketing use of somatropin products.
- Fluid retention in adults (clinically manifesting as edema, arthralgia, myalgia, nerve compression syndromes including carpal tunnel syndrome/paraesthesias) may frequently occur and is usually transient and dose-dependent.
- Hypoadrenalism: Patients who have or are at risk for pituitary hormone deficiency(s) may be at risk for reduced serum cortisol levels and/or unmasking of central (secondary) hypoadrenalism. In addition, patients treated with glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses following initiation of Norditropin® treatment.
- Hypothyroidism if undiagnosed/untreated, may prevent an optimal response to Norditropin®, in particular, the growth response in pediatric patients. In patients with GHD, central (secondary) hypothyroidism may first become evident or worsen during somatropin treatment. Periodic thyroid function tests and thyroid hormone replacement therapy should be initiated or adjusted when indicated.
- Slipped capital femoral epiphysis in pediatric patients may occur more frequently in patients with endocrine disorders or in patients undergoing rapid growth. Pediatric patients with the onset of a limp or complaints of hip or knee pain should be evaluated.
- Progression of preexisting scoliosis in pediatric patients can occur in patients who experience rapid growth. Patients with a history of scoliosis should be monitored for progression.
- Pancreatitis: Cases of pancreatitis have been reported. Pancreatitis should be considered in any patient who develops persistent severe abdominal pain.
- Lipoatrophy: Tissue atrophy may result when somatropin is administrated subcutaneously at the same site over a long period of time. Rotate injection sites when administering Norditropin® to reduce this risk.
Adverse Reactions
- Other common adverse reactions in adults and pediatric patients include: upper respiratory infection, fever, pharyngitis, headache, otitis media, edema, arthralgia, paresthesia, myalgia, peripheral edema, flu syndrome, and impaired glucose tolerance
Drug Interactions
- Glucocorticoids: Patients treated with glucocorticoid for hypoadrenalism may require an increase in their maintenance or stress doses following initiation of Norditropin®
- Pharmacologic Glucocorticoid Therapy and Supraphysiologic Glucocorticoid Treatment: Adjust glucocorticoid replacement dosing in pediatric patients receiving glucocorticoid treatment to avoid both hypoadrenalism and an inhibitory effect on growth
- Cytochrome P450-Metabolized Drugs: Norditropin® may alter the clearance. Monitor carefully if used with Norditropin®
- Oral Estrogen: Larger doses of Norditropin® may be required
- Insulin and/or Other Hypoglycemic Agents: Dose adjustment of insulin or hypoglycemic agent may be required
Use in Specific Populations
- Pregnancy and Nursing Mothers: There are limited data with somatropin use in pregnant women and nursing mothers to inform a drug-associated risk for adverse developmental outcomes.
- Geriatric Use: The safety and effectiveness in patients aged 65 and over has not been evaluated in clinical studies.
Please click here for Norditropin® Prescribing Information.
Important Safety Information for Norditropin®
Contraindications
Norditropin® is contraindicated in patients with:
- Acute critical illness after open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure due to the risk of increased mortality with use of pharmacologic doses of somatropin
- Pediatric patients with Prader-Willi syndrome who are severely obese, have a history of upper airway obstruction or sleep apnea, or have severe respiratory impairment due to the risk of sudden death
- Active Malignancy
- Hypersensitivity to Norditropin® or any of its excipients. Systemic hypersensitivity reactions have been reported with postmarketing use of somatropin products
- Active proliferative or severe non-proliferative diabetic retinopathy
- Pediatric patients with closed epiphyses
Indications and Usage
Norditropin® (somatropin) injection is indicated for the treatment of pediatric patients with:
- growth failure due to inadequate secretion of endogenous growth hormone (GH)
- short stature associated with Noonan syndrome,
- short stature associated with Turner syndrome,
- short stature born small for gestational age (SGA) with no catch-up growth by age 2 to 4 years of age
- Idiopathic Short Stature (ISS), height standard deviation score (SDS) <-2.25, and associated with growth rates unlikely to permit attainment of adult height in the normal range
- growth failure due to Prader-Willi syndrome (PWS)
Norditropin® is also indicated for the replacement of endogenous GH in adults with growth hormone deficiency (GHD)
Important Safety Information
Contraindications
Norditropin® is contraindicated in patients with:
- Acute critical illness after open heart surgery, abdominal surgery or multiple accidental trauma, or those with acute respiratory failure due to the risk of increased mortality with use of pharmacologic doses of somatropin
- Pediatric patients with Prader-Willi syndrome who are severely obese, have a history of upper airway obstruction or sleep apnea, or have severe respiratory impairment due to the risk of sudden death
- Active Malignancy
- Hypersensitivity to Norditropin® or any of its excipients. Systemic hypersensitivity reactions have been reported with postmarketing use of somatropin products
- Active proliferative or severe non-proliferative diabetic retinopathy
- Pediatric patients with closed epiphyses
Warnings and Precautions
- Increased mortality in patients with acute critical illness due to complications following open heart or abdominal surgery or multiple accidental trauma, or those with respiratory failure has been reported.
- Sudden death in pediatric patients with Prader-Willi Syndrome has been reported after initiating treatment with somatropin with one or more of the following risk factors: severe obesity, history of upper airway obstruction or sleep apnea, or unidentified respiratory infection. Evaluate patients for signs of upper airway obstruction and sleep apnea before initiation of treatment.
- Increased risk of neoplasms: Monitor patients with preexisting tumors for progression or recurrence. In childhood cancer survivors who were treated with radiation to the brain/head for their first neoplasm and who developed subsequent GHD and were treated with somatropin, an increased risk of a second neoplasm, in particular meningiomas, has been reported. Pediatric patients with certain rare genetic causes of short stature have an increased risk of developing malignancies and should be carefully monitored for development of neoplasms. Monitor patients carefully for increased growth, or potential malignant changes, of preexisting nevi.
- Glucose intolerance and diabetes mellitus: Treatment with somatropin may decrease insulin sensitivity, particularly at higher doses. New-onset type 2 diabetes mellitus has been reported. Monitor glucose levels in all patients. Doses of concurrent antidiabetic drugs may require adjustment.
- Intracranial hypertension has been reported in a small number of patients, usually within the first 8 weeks of somatropin treatment. Funduscopic examination should be performed before initiating treatment and periodically thereafter.
- Severe hypersensitivity: Serious systemic hypersensitivity reactions including anaphylactic reactions and angioedema have been reported with postmarketing use of somatropin products.
- Fluid retention in adults (clinically manifesting as edema, arthralgia, myalgia, nerve compression syndromes including carpal tunnel syndrome/paraesthesias) may frequently occur and is usually transient and dose-dependent.
- Hypoadrenalism: Patients who have or are at risk for pituitary hormone deficiency(s) may be at risk for reduced serum cortisol levels and/or unmasking of central (secondary) hypoadrenalism. In addition, patients treated with glucocorticoid replacement for previously diagnosed hypoadrenalism may require an increase in their maintenance or stress doses following initiation of Norditropin® treatment.
- Hypothyroidism if undiagnosed/untreated, may prevent an optimal response to Norditropin®, in particular, the growth response in pediatric patients. In patients with GHD, central (secondary) hypothyroidism may first become evident or worsen during somatropin treatment. Periodic thyroid function tests and thyroid hormone replacement therapy should be initiated or adjusted when indicated.
- Slipped capital femoral epiphysis in pediatric patients may occur more frequently in patients with endocrine disorders or in patients undergoing rapid growth. Pediatric patients with the onset of a limp or complaints of hip or knee pain should be evaluated.
- Progression of preexisting scoliosis in pediatric patients can occur in patients who experience rapid growth. Patients with a history of scoliosis should be monitored for progression.
- Pancreatitis: Cases of pancreatitis have been reported. Pancreatitis should be considered in any patient who develops persistent severe abdominal pain.
- Lipoatrophy: Tissue atrophy may result when somatropin is administrated subcutaneously at the same site over a long period of time. Rotate injection sites when administering Norditropin® to reduce this risk.
Adverse Reactions
- Other common adverse reactions in adults and pediatric patients include: upper respiratory infection, fever, pharyngitis, headache, otitis media, edema, arthralgia, paresthesia, myalgia, peripheral edema, flu syndrome, and impaired glucose tolerance
Drug Interactions
- Glucocorticoids: Patients treated with glucocorticoid for hypoadrenalism may require an increase in their maintenance or stress doses following initiation of Norditropin®
- Pharmacologic Glucocorticoid Therapy and Supraphysiologic Glucocorticoid Treatment: Adjust glucocorticoid replacement dosing in pediatric patients receiving glucocorticoid treatment to avoid both hypoadrenalism and an inhibitory effect on growth
- Cytochrome P450-Metabolized Drugs: Norditropin® may alter the clearance. Monitor carefully if used with Norditropin®
- Oral Estrogen: Larger doses of Norditropin® may be required
- Insulin and/or Other Hypoglycemic Agents: Dose adjustment of insulin or hypoglycemic agent may be required
Use in Specific Populations
- Pregnancy and Nursing Mothers: There are limited data with somatropin use in pregnant women and nursing mothers to inform a drug-associated risk for adverse developmental outcomes.
- Geriatric Use: The safety and effectiveness in patients aged 65 and over has not been evaluated in clinical studies.
Please click here for Norditropin® Prescribing Information.

